|
 |
 |
 |
 |
 |
 |
| Selected Media Reports | ||||||
Lee Buckler
|
Jay Bhogal
|
Thomas Bold
|
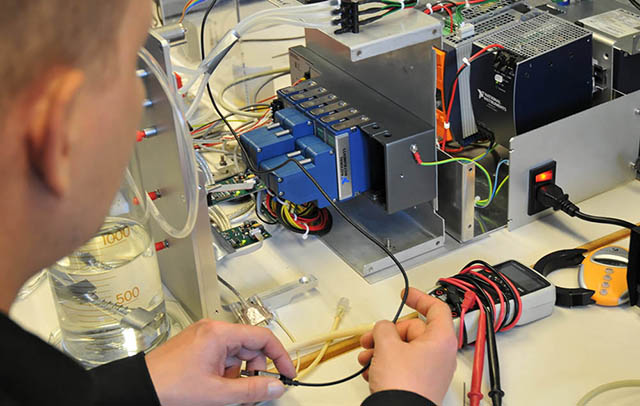
Innovative Thinking and Practical Engineering
We work on: the cutting edge of applied human cell, stem cell and progenitor cell sciences and associated bioengineering for regenerative medicine therapies.
We build: first-of-their-kind medical devices and cell technologies.
We develop: enhancements for commercial products for clients who seek to streamline their companies` regulatory-, production-, logistics-, and service strategies, to allow for enhanced marketing. This includes novel medical devices, and cell product technologies.
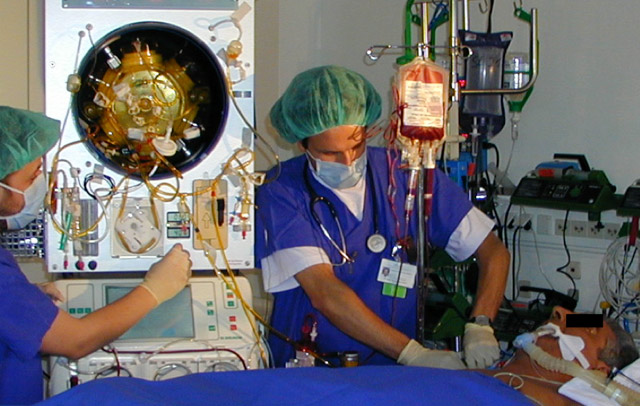
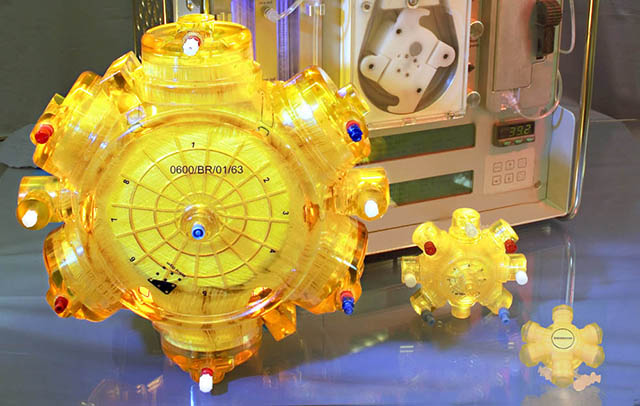
Liver Support Bioreactor Device
Skin Cell Spray Grafting Device
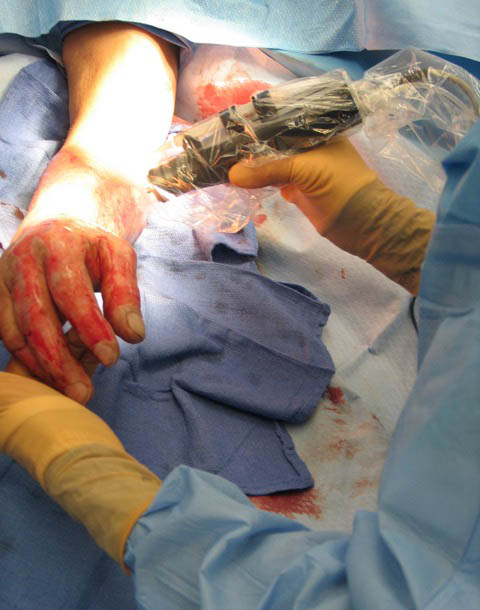
Clinical Study Prototyping
Our long-standing history of clinical translation project work and device prototyping that have been successfully used in clinical studies is offered to our clients.
Our team includes experts in medical device development, cell technologies; prototyping, production and regulatory affairs; but also clinical expertise in medicine and surgery.
Our CEO is rooted in clinical medicine and intensive care, our CTO is rooted in bioengineering, and our founder is an MD with hands-on experience in surgery and intensive care, a PhD in Experimental Surgery and also a PhD in Bioengineering, and is deeply rooted in university research on human stem and progenitor cell biology.
We therefore can offer a unique combination of expertise for clinical study prototyping.
Skin Cell Sprayer, for RenovaCare, NY
Resources
Our resources include clean room R&D facility, clean room prototyping facility, electronic engineering lab, mechanical engineering lab, IT lab, medical device lab, polymer lab, membrane lab, bioengineering lab, client project management, client regulatory service, IRB/ IDE/ 510(k) submission support, client QMS development and service, analyses and testing, clinical study support.

Current R&D Pipeline
Our current R&D pipeline for clinical study-ready devices includes: dermal cell injectors, epidermal/ dermal cell spray deposition devices, culture technology for drug development, bioreactors for liver support and blood cell expansion, active wound dressing technologies, cell isolation technologies, cell production technologies.
Our R&D Service Offers
Our R&D service for clinical study prototyping offers: consulting, medical device development that includes electrical and mechanical and IT engineering, stem cell and progenitor cell technologies, device prototyping and clinical study scale production.

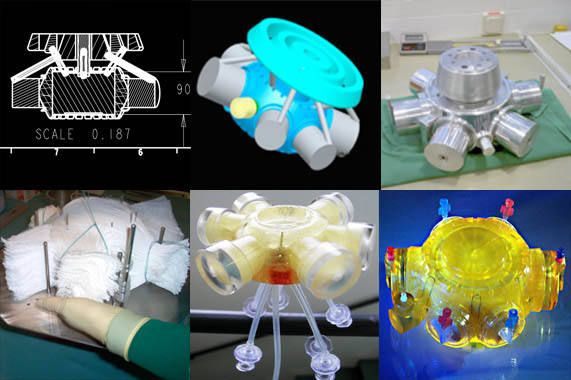
We believe, that 3D Tissue Restructuring in our Bio Reactors is really cool!

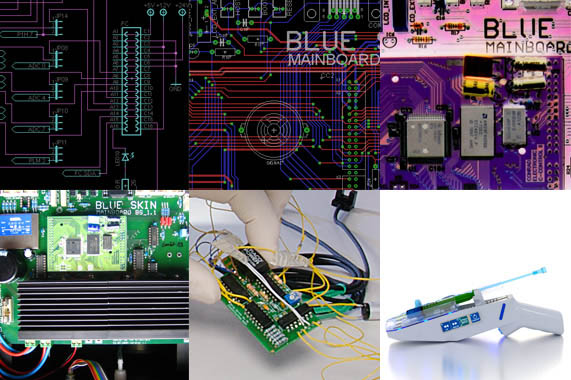
Expertise
We have a history of commercial and technical success stories.
Our inventions, projects and devices have been featured in international world-class scientific journals and news outlets (see the banners on this page).
StemCell Systems - How medical technologies and devices trigger the body´s natural ability to heal itself.
…about Regenerative Medicine and Stem Cells


Network
Located in Berlin, Germany and rooted in German engineering and biomedical research, we are embedded in a network of regional and international experts in Europe and the USA. Our partners and clients are highly-credible research institutions, government agencies, and corporations (see banner).

StemCell Systems GmbH
Airport Berlin-Tempelhof
Hangar 6.c
Tempelhofer Damm 45
12101 Berlin, Germany
fon: +49 (0)30 / 6290188-0
fax: +49 (0)30 / 6290188-19
e-mail:
office@stemcell-systems.com
www.stemcell-systems.com
CEO: Reinhard Bornemann, MD, PhD
CTO and Chief Engineer, Registered Manager (Prokurist): Frank Schubert
Owners: R. Bornemann, MD, PhD and J. Gerlach, MD, PhD
Registered: Amtsgericht Berlin-Charlottenburg, Germany HRB 114204B
ltd. liability company (GmbH)
Bank: Deutsche Apotheker- und Ärztebank Berlin
IBAN: DE50 3006 0601 0006 5308 50
BIC: DAAEDEDDXXX
German Tax No: 29/450/01217
Umsatzsteuer Identification No: DE 143446861 DUNS No: 340482749
Find us:
- Find Us by Car
- Find Us by Public Transport
- To Hangar 6.c
- Google Maps
PRICE LIST R&D / CONTRACTING SERVICES •
TERMS AND CONDITIONS • IMPORTANT RESTRICTIONS •
WIRE TRANSFER
MELS •
EARLIER PAGE
Disclaimer: Responsible for the content of this site according to § 6 MDStV (the German Mediendienststaatsvertrag): StemCell Systems GmbH Liability disclosure: Despite our careful inspection we are not liable for the contents of external links. The contents of the linked pages are the sole responsibility of the operator. | Data Privacy Policy | Datenschutzerklärung
Webdesign form@